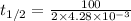Chemistry, 11.02.2020 21:27 litzyguzman13
In a zero order reaction, it takes 342 seconds for 75% of a hypothetical reaction to decompose. Determine the half-life t1/2} in units of seconds. Do not enter units with your numerical answer.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
In a zero order reaction, it takes 342 seconds for 75% of a hypothetical reaction to decompose. Dete...
Questions





Mathematics, 04.07.2020 14:01









Mathematics, 04.07.2020 14:01


Mathematics, 04.07.2020 14:01


English, 04.07.2020 14:01

Mathematics, 04.07.2020 14:01


![\ln [A]=-kt+\ln [A_o]](/tpl/images/0507/1434/bdc3f.png)
![[A_o]](/tpl/images/0507/1434/dc622.png) = let initial concentration = 100
= let initial concentration = 100![[A]](/tpl/images/0507/1434/6aa06.png) = final concentration = 25
= final concentration = 25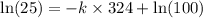
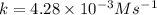
![t_{1/2}=\frac{[A_o]}{2k}](/tpl/images/0507/1434/b5b11.png)