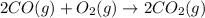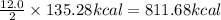Chemistry, 11.02.2020 20:55 7letters22
Carbon monoxide reacts with oxygen to form carbon dioxide by the following reaction: 2 CO(g) + O2(g) → 2 CO2(g)∆H for this reaction is −135.28 kcal. How much heat would be released if 12.0 moles of carbon monoxide reacted with sufficient oxygen to produce carbon dioxide? Use only the information provided in this question. 1. 270.56 kcal 2. 1623.36 kcal 3. 811.68 kcal 4. 405.84 kcal 5. 541.12 kcal 6. 135.28 kcal

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

You know the right answer?
Carbon monoxide reacts with oxygen to form carbon dioxide by the following reaction: 2 CO(g) + O2(g)...
Questions

English, 01.10.2019 17:00


Mathematics, 01.10.2019 17:00

Mathematics, 01.10.2019 17:00




Chemistry, 01.10.2019 17:00


Mathematics, 01.10.2019 17:00

Mathematics, 01.10.2019 17:00

Mathematics, 01.10.2019 17:00



Mathematics, 01.10.2019 17:00


Biology, 01.10.2019 17:00


Mathematics, 01.10.2019 17:00

Spanish, 01.10.2019 17:00