
Chemistry, 11.02.2020 20:55 brummy309506
Nitrogen and hydrogen react to form ammonia according to the following balanced equation: N2(g) + 3H2(g) → 2NH3(g) Calculate the number of moles of hydrogen required to react with 0.0763 mole of nitrogen, and the number of moles of ammonia that will form.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1

You know the right answer?
Nitrogen and hydrogen react to form ammonia according to the following balanced equation: N2(g) + 3H...
Questions

Mathematics, 01.04.2021 16:30

History, 01.04.2021 16:30



Mathematics, 01.04.2021 16:30

Computers and Technology, 01.04.2021 16:30


English, 01.04.2021 16:30

Biology, 01.04.2021 16:30



Spanish, 01.04.2021 16:30



Social Studies, 01.04.2021 16:30





Mathematics, 01.04.2021 16:30

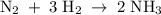
 3 moles of Hydrogen
3 moles of Hydrogen

