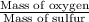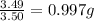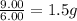Chemistry, 11.02.2020 20:15 davidsouth444
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. When samples of these were decomposed the sulfur dioxide produced 3.49g oxygen and 3.50g sulfur, while the sulfur trioxide produced 9.00g oxygen and 6.00g sulfur. A) Calculate the mass of oxygen per gram of sulfur for sulfur dioxide. B) Calculate the mass of oxygen per gram of sulfur for sulfur trioxide.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

You know the right answer?
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. When samples of these were decompose...
Questions

Mathematics, 11.03.2021 17:50

Business, 11.03.2021 17:50

Mathematics, 11.03.2021 17:50



Mathematics, 11.03.2021 17:50

Mathematics, 11.03.2021 17:50

Mathematics, 11.03.2021 17:50



Mathematics, 11.03.2021 17:50

Health, 11.03.2021 17:50

Chemistry, 11.03.2021 17:50


Business, 11.03.2021 17:50

Mathematics, 11.03.2021 17:50



Mathematics, 11.03.2021 17:50

Social Studies, 11.03.2021 17:50