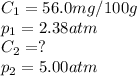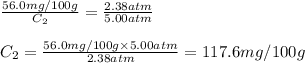Chemistry, 11.02.2020 19:23 Whitehouse9
At a certain temperature, the solubility of N₂ gas in water at 2.38 atm is 56.0 mg of N₂ gas/100 g water. Calculate the solubility of N₂ gas in water, at the same temperature, if the partial pressure of N₂ gas over the solution is increased from 2.38 atm to 5.00 atm.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2


Chemistry, 23.06.2019 02:10
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2
You know the right answer?
At a certain temperature, the solubility of N₂ gas in water at 2.38 atm is 56.0 mg of N₂ gas/100 g w...
Questions







Computers and Technology, 24.07.2019 21:00


History, 24.07.2019 21:00




Social Studies, 24.07.2019 21:00

English, 24.07.2019 21:00



Chemistry, 24.07.2019 21:00

Mathematics, 24.07.2019 21:00


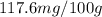
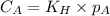
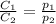
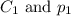 are the initial concentration and partial pressure of nitrogen gas
are the initial concentration and partial pressure of nitrogen gas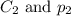 are the final concentration and partial pressure of nitrogen gas
are the final concentration and partial pressure of nitrogen gas