
Chemistry, 11.02.2020 18:45 Theresab2021
The decomposition of N2O5 in solution in carbon tetrachloride proceeds via the reaction 2 N2O5(soln) → 4 NO2(soln) + O2(soln) The reaction is first order and has a rate constant of 4.82 × 10-3 s-1 at 64°C. If the reaction is initiated with 0.085 mol in a 1.00-L vessel, how many moles remain after 151 s?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 07:00
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2

Chemistry, 23.06.2019 13:00
How many grams of oxygen gas will react completely with a block of calcium metal that is 3.0 cm by 3.5 cm by 4.2 cm, if the density of calcium is 1.55 g/ml? show all steps of your calculation as well as the final answer.
Answers: 3
You know the right answer?
The decomposition of N2O5 in solution in carbon tetrachloride proceeds via the reaction 2 N2O5(soln)...
Questions

Mathematics, 16.12.2020 19:30


Biology, 16.12.2020 19:30


Social Studies, 16.12.2020 19:30


Chemistry, 16.12.2020 19:30

English, 16.12.2020 19:30





Mathematics, 16.12.2020 19:30


Chemistry, 16.12.2020 19:30




Mathematics, 16.12.2020 19:30

Mathematics, 16.12.2020 19:30

![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0506/7634/f1041.png)
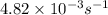
![[A_o]](/tpl/images/0506/7634/dc622.png) = initial amount of the reactant = 0.085 moles
= initial amount of the reactant = 0.085 moles![4.82\times 10^{-3}=\frac{2.303}{151}\log\frac{0.085}{[A]}](/tpl/images/0506/7634/8f69f.png)
![[A]=0.041moles](/tpl/images/0506/7634/e3fb9.png)


