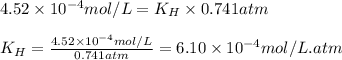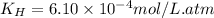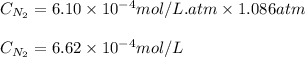Chemistry, 11.02.2020 05:05 humblemalak
The solubility of nitrogen, N2, in water is 4.52 ✕ 10−4 mol/L at 0°C when the nitrogen pressure above water is 0.741 atm. Calculate the solubility of nitrogen in water when the partial pressure of nitrogen above water is 1.086 atm at 0°C?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1
You know the right answer?
The solubility of nitrogen, N2, in water is 4.52 ✕ 10−4 mol/L at 0°C when the nitrogen pressure abov...
Questions

Mathematics, 02.12.2020 23:00



Spanish, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00

History, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00



Mathematics, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00

History, 02.12.2020 23:00


Mathematics, 02.12.2020 23:00

Mathematics, 02.12.2020 23:00

Physics, 02.12.2020 23:00

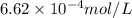
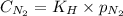
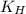 = Henry's constant = ?
= Henry's constant = ?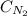 = molar solubility of nitrogen gas =
= molar solubility of nitrogen gas = 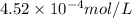
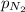 = partial pressure of nitrogen gas = 0.741 atm
= partial pressure of nitrogen gas = 0.741 atm