
Chemistry, 11.02.2020 04:25 Andychulo7809
Calculate the amount of heat (in kJ) required to convert 62.6 g of water to steam at 100°C. (The molar heat of vaporization of water is 40.79 kJ/mol.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1


Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
Calculate the amount of heat (in kJ) required to convert 62.6 g of water to steam at 100°C. (The mol...
Questions

Mathematics, 13.10.2019 09:10


Mathematics, 13.10.2019 09:10





Mathematics, 13.10.2019 09:10


Mathematics, 13.10.2019 09:10



Mathematics, 13.10.2019 09:10


English, 13.10.2019 09:10

English, 13.10.2019 09:10

Biology, 13.10.2019 09:10



Health, 13.10.2019 09:10

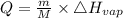
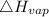 = 40.79 kJ/mol
= 40.79 kJ/mol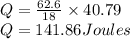
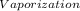 ·m where Q = required heat energy,
·m where Q = required heat energy, 

