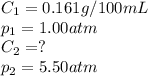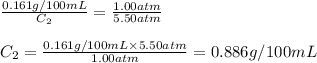The solubility of carbon dioxide in water is 0.161 g CO2in 100 mL of water at 20ºC and 1.00 atmCO2. A soft drink is carbonated with carbon dioxide gas at 5.50 atm pressure. What is the solubility of carbon dioxide in water at this pressure (assume temperature in all cases is 20ºC).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2
You know the right answer?
The solubility of carbon dioxide in water is 0.161 g CO2in 100 mL of water at 20ºC and 1.00 atmCO2....
Questions


Computers and Technology, 13.11.2020 18:00

English, 13.11.2020 18:00




Chemistry, 13.11.2020 18:00

Mathematics, 13.11.2020 18:00

English, 13.11.2020 18:00


Chemistry, 13.11.2020 18:00

Mathematics, 13.11.2020 18:00



Mathematics, 13.11.2020 18:00


Mathematics, 13.11.2020 18:00


Arts, 13.11.2020 18:00

Physics, 13.11.2020 18:00

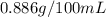
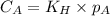
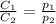
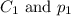 are the initial concentration and partial pressure of carbon dioxide
are the initial concentration and partial pressure of carbon dioxide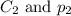 are the final concentration and partial pressure of carbon dioxide
are the final concentration and partial pressure of carbon dioxide