
Chemistry, 11.02.2020 03:23 kookycookiefanx
Suppose a certain biologically important reaction is quite slow at physiological temperature (37 °C) in the absence of a catalyst. Assuming that the frequency factor remains the same, by how much must an enzyme lower the activation energy of the reaction to achieve a million- fold (1.0 x 106) increase in the reaction rate constant?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
You know the right answer?
Suppose a certain biologically important reaction is quite slow at physiological temperature (37 °C)...
Questions




Chemistry, 27.09.2020 14:01

Mathematics, 27.09.2020 14:01

Mathematics, 27.09.2020 14:01

English, 27.09.2020 14:01


Mathematics, 27.09.2020 14:01


Biology, 27.09.2020 14:01

Mathematics, 27.09.2020 14:01

Chemistry, 27.09.2020 14:01


Mathematics, 27.09.2020 14:01


Chemistry, 27.09.2020 14:01

English, 27.09.2020 14:01


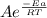
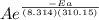
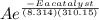
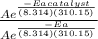 = 1.0 x 10⁶ by taking natural logarithm of both sides, we have
= 1.0 x 10⁶ by taking natural logarithm of both sides, we have


