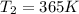Chemistry, 11.02.2020 01:16 serenityparish
A certain reaction has an activation energy of 28.90 kJ / mol. At what Kelvin temperature will the reaction proceed 5.00 times faster than it did at 313 K?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2


Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
You know the right answer?
A certain reaction has an activation energy of 28.90 kJ / mol. At what Kelvin temperature will the r...
Questions

Biology, 09.12.2020 02:00


Mathematics, 09.12.2020 02:00


Biology, 09.12.2020 02:00

Chemistry, 09.12.2020 02:00

Mathematics, 09.12.2020 02:00

History, 09.12.2020 02:00





Mathematics, 09.12.2020 02:00




Mathematics, 09.12.2020 02:00



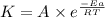
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0505/7015/6d953.png)
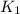 = rate constant at
= rate constant at 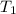 = 1.00
= 1.00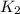 = rate constant at
= rate constant at 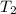 = 5.00
= 5.00 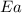 = activation energy for the reaction = 28.90 kJ/mol= 28900 j/mol
= activation energy for the reaction = 28.90 kJ/mol= 28900 j/mol![\log (\frac{5.00}{1.00})=\frac{28900}{2.303\times 8.314J/mole.K}[\frac{1}{313K}-\frac{1}{T_2K}]](/tpl/images/0505/7015/9930b.png)
![0.69=\frac{28900}{2.303\times 8.314J/mole.K}[\frac{1}{313K}-\frac{1}{T_2K}]](/tpl/images/0505/7015/90df4.png)