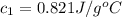Chemistry, 11.02.2020 01:14 treseanthegreat
A piece of unknown metal with mass 30 g is heated to 110.0 °C and dropped into 100.0 g of water at 20.0 °C. The final temperature of the system is 25.0 °C. Determine the specific heat of the metal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1
You know the right answer?
A piece of unknown metal with mass 30 g is heated to 110.0 °C and dropped into 100.0 g of water at 2...
Questions


Mathematics, 17.09.2019 16:30


Mathematics, 17.09.2019 16:30

Mathematics, 17.09.2019 16:30

Mathematics, 17.09.2019 16:30

Mathematics, 17.09.2019 16:30

English, 17.09.2019 16:30


Mathematics, 17.09.2019 16:30

Chemistry, 17.09.2019 16:30

English, 17.09.2019 16:30

Mathematics, 17.09.2019 16:30

Mathematics, 17.09.2019 16:30




Business, 17.09.2019 16:30


English, 17.09.2019 16:30

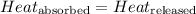
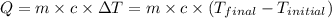
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0505/6952/09236.png) ......(1)
......(1)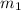 = mass of metal = 30 g
= mass of metal = 30 g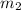 = mass of water = 100 g
= mass of water = 100 g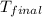 = final temperature = 25°C
= final temperature = 25°C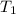 = initial temperature of metal = 110°C
= initial temperature of metal = 110°C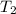 = initial temperature of water = 20.0°C
= initial temperature of water = 20.0°C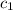 = specific heat of metal = ?
= specific heat of metal = ?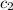 = specific heat of water = 4.186 J/g°C
= specific heat of water = 4.186 J/g°C![30\times c_1\times (25-110)=-[100\times 4.186\times (25-20)]](/tpl/images/0505/6952/ee389.png)