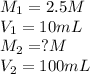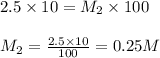Chemistry, 10.02.2020 21:51 cookies1164
What is the concentration of a 1:10 dilution of a 2.5 M solution of NaCl? How would you prepare exactly 100 ml of such a solution?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1


Chemistry, 23.06.2019 15:30
The amount of iron in ore can be quantitatively determined by titrating a solution of the unknown with a standard solution of dichromate, cr2o72−. the net ionic equation is 6fe2+(aq)+cr2o72−(aq)+14h+(aq)→6fe3+(aq)+2cr3+(aq)+7h2o(aq) part a the titration of 25.0 ml of an iron(ii) solution required 18.0 ml of a 0.230 m solution of dichromate to reach the equivalence point. what is the molarity of the iron(ii) solution?
Answers: 1
You know the right answer?
What is the concentration of a 1:10 dilution of a 2.5 M solution of NaCl? How would you prepare exac...
Questions

Medicine, 02.12.2021 23:00

Mathematics, 02.12.2021 23:00







Social Studies, 02.12.2021 23:00

History, 02.12.2021 23:00





Chemistry, 02.12.2021 23:00

Mathematics, 02.12.2021 23:00

Social Studies, 02.12.2021 23:00



Social Studies, 02.12.2021 23:00

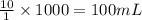
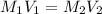
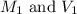 are the molarity and volume of the concentrated NaCl solution
are the molarity and volume of the concentrated NaCl solution
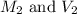 are the molarity and volume of diluted NaCl solution
are the molarity and volume of diluted NaCl solution