15 points!
I have a lab to complete and I'd like help on some yields!
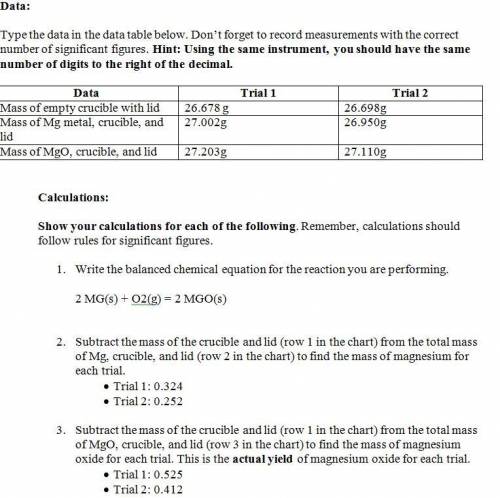
1. Mag...

15 points!
I have a lab to complete and I'd like help on some yields!
1. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
• Trial 1:
• Trial 2:
2. Determine the percent yield of MgO for your experiment for each trial.
• Trial 1:
• Trial 2:
Attached image: data

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
Questions


Mathematics, 25.04.2020 01:23












World Languages, 25.04.2020 01:23

History, 25.04.2020 01:23


Mathematics, 25.04.2020 01:23


Spanish, 25.04.2020 01:24



