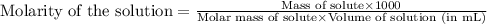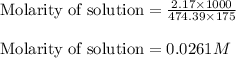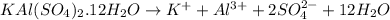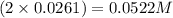Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
What is the molarity of SO4^2- in a solution prepared by mixing 2.17 g of alum, KAl(SO4)2•12H2O, wit...
Questions

Mathematics, 20.10.2020 03:01




Mathematics, 20.10.2020 03:01

Physics, 20.10.2020 03:01

Social Studies, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01


Spanish, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01

Social Studies, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01

Mathematics, 20.10.2020 03:01

Computers and Technology, 20.10.2020 03:01


Mathematics, 20.10.2020 03:01

English, 20.10.2020 03:01