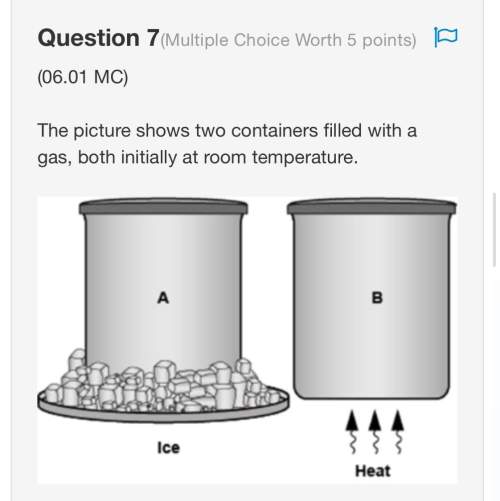
Chemistry, 10.02.2020 21:03 cshopholic4921
A chemical equilibrium between gaseous reactants and products is shown.
N2(g) + 3H2(g) ⇌ 2NH3(g)
How will the reaction be affected if the pressure on the system is decreased? (3 points)
It will shift toward the reactant side as there is lower pressure on the reactant side.
It will shift toward the product side as there is higher pressure on the product side.
It will shift toward the reactant side as there are a greater number of moles of gas on the reactant side.
It will shift toward the product side as there are a fewer number of moles of gas on the product side.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Pbco3 –> pbo+ co2. how many liters of carbon dioxide gas is produced from the decomposition of 32 grams of lead (ll) carbonate?
Answers: 1


Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
You know the right answer?
A chemical equilibrium between gaseous reactants and products is shown.
N2(g) + 3H2(g) ⇌...
N2(g) + 3H2(g) ⇌...
Questions





Mathematics, 18.08.2019 15:30


English, 18.08.2019 15:30

English, 18.08.2019 15:30




Mathematics, 18.08.2019 15:30


Biology, 18.08.2019 15:30

Mathematics, 18.08.2019 15:30


History, 18.08.2019 15:30

Mathematics, 18.08.2019 15:30

Chemistry, 18.08.2019 15:30

History, 18.08.2019 15:30