Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
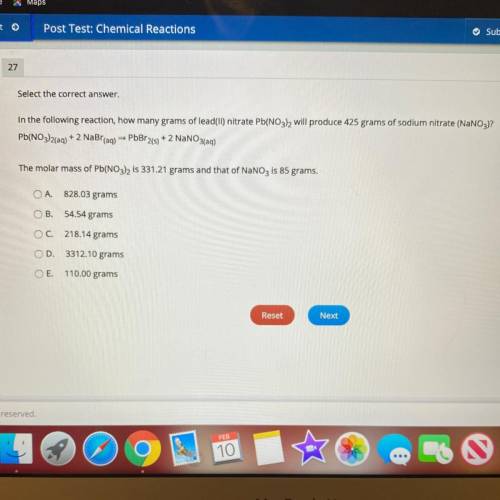
In the following reaction, how many grams of lead(II) nitrate Pb(NO3), will produce 425 grams of sod...
Questions





Mathematics, 21.10.2020 19:01


Mathematics, 21.10.2020 19:01

Mathematics, 21.10.2020 19:01


Mathematics, 21.10.2020 19:01

Mathematics, 21.10.2020 19:01

Spanish, 21.10.2020 19:01


Physics, 21.10.2020 19:01



Mathematics, 21.10.2020 19:01


Mathematics, 21.10.2020 19:01

English, 21.10.2020 19:01