
Chemistry, 10.02.2020 20:02 jessicaou2005
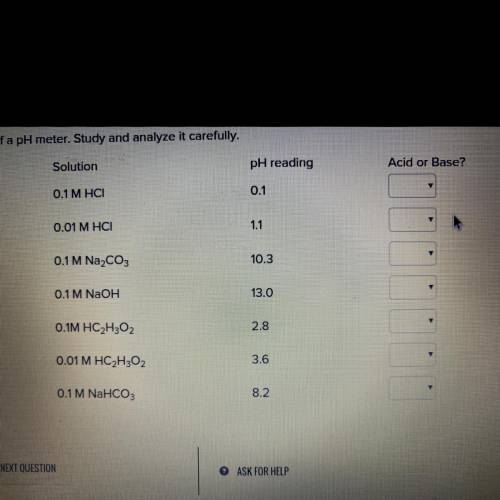
The pH of a solution can be determined with an instrument called a pH meter. The following data was collected with the
use of a pH meter. Study and analyze it carefully.
Solution 0.1 M HCI
0.01 M HCI
0.1 M Na2CO3
0.1 M NaOH
O.1M HC 2 H 3 O 2
0.01 M HC 2 H 3 O 2
0.1 M NaHCO 3
pH reading
0.1
1.1
10.3
13.0
2.8
3.6
8.2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1


Chemistry, 23.06.2019 09:00
Which question could be best answered using the process of scientific inquiry? do different plates have different rock compositions? why did it take so long to develop the theory of plate tectonics? what are different cultural myths caused by plate tectonics? do plates move intentionally to cause volcanic eruptions?
Answers: 3
You know the right answer?
The pH of a solution can be determined with an instrument called a pH meter. The following data was...
Questions

Physics, 07.07.2019 23:00


Arts, 07.07.2019 23:00


Mathematics, 07.07.2019 23:00

Mathematics, 07.07.2019 23:00

Mathematics, 07.07.2019 23:00


Mathematics, 07.07.2019 23:00



Biology, 07.07.2019 23:00


Mathematics, 07.07.2019 23:00

Chemistry, 07.07.2019 23:00


Mathematics, 07.07.2019 23:00

Computers and Technology, 07.07.2019 23:00




