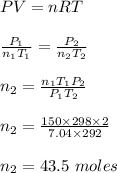Chemistry, 10.02.2020 05:39 Sonicawesomeness
A steel cylinder contains 150.0 mol argon gas at a temperature of 25°C and a pressure of 7.04 MPa. After some argon has been used, the pressure is 2.00 MPa at a temperature of 19°C. What mass (g) of argon remains in the cylinder?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
A steel cylinder contains 150.0 mol argon gas at a temperature of 25°C and a pressure of 7.04 MPa. A...
Questions



Mathematics, 04.03.2021 14:00

History, 04.03.2021 14:00



Mathematics, 04.03.2021 14:00

Computers and Technology, 04.03.2021 14:00

Physics, 04.03.2021 14:00

Mathematics, 04.03.2021 14:00

Mathematics, 04.03.2021 14:00

Mathematics, 04.03.2021 14:00

Mathematics, 04.03.2021 14:00






Mathematics, 04.03.2021 14:00

English, 04.03.2021 14:00