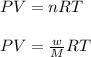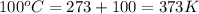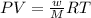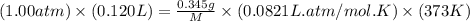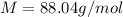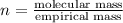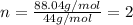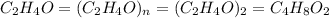Chemistry, 05.02.2020 21:41 2020seogang
Analysis of a volatile liquid showed that it is 54.5% carbon, 9.1% hydrogen, and 36.4% oxygen by mass. a separate 0.345-gram sample of its vapor occupied 120. ml at 100.°c and 1.00 atm. what is the molecular formula for the compound?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

You know the right answer?
Analysis of a volatile liquid showed that it is 54.5% carbon, 9.1% hydrogen, and 36.4% oxygen by mas...
Questions








English, 05.03.2021 22:50


Engineering, 05.03.2021 22:50


Mathematics, 05.03.2021 22:50

Chemistry, 05.03.2021 22:50

History, 05.03.2021 22:50

Mathematics, 05.03.2021 22:50

Spanish, 05.03.2021 22:50


Mathematics, 05.03.2021 22:50


Chemistry, 05.03.2021 22:50

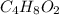
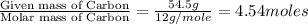
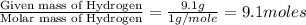
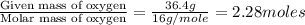
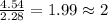
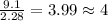
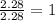
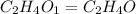
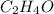 = 2(12) + 4(1) + 16 = 44 g/eq.
= 2(12) + 4(1) + 16 = 44 g/eq.