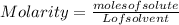The equation used to calculate the change in freezing point (δtf) of a substance is:
δtf = k...

The equation used to calculate the change in freezing point (δtf) of a substance is:
δtf = kfm
where kf is the freezing point depression constant and m is the molality of the solution. which of the statements explains why molality is used instead of molarity in this equation?
a. molality does not appear in many equations, so it is used here to distinguish this equation from other similar ones.
b. as the temperature of a solution changes, its volume will also change, which will affect its molarity but not its molality.
c. in solutions, moles are not directly related to grams and the freezing point of a solution is dependent solely on the number of grams of solute.
d. the equation was originally published with m as a typo, rather than m, but the values are close enough that the equation is still valid.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2


Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1
You know the right answer?
Questions

Social Studies, 06.04.2021 20:50


English, 06.04.2021 20:50



Mathematics, 06.04.2021 20:50

Mathematics, 06.04.2021 20:50




History, 06.04.2021 20:50



Mathematics, 06.04.2021 20:50

Chemistry, 06.04.2021 20:50

Mathematics, 06.04.2021 20:50



Mathematics, 06.04.2021 20:50