Chemistry, 04.02.2020 05:51 elijahjacksonrp6z2o7
How much energy must be removed from a 125 g sample of benzene (molar mass= 78.11 g/mol) at 425.0 k to liquify the sample and lower the temperature to 335.0 k? the following physical data may be useful.
hvap = 33.9 kj/molhfus = 9.8 kj/mol
cliq = 1.73 j/g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
How much energy must be removed from a 125 g sample of benzene (molar mass= 78.11 g/mol) at 425.0 k...
Questions

Health, 26.07.2019 09:30


Geography, 26.07.2019 09:40

Mathematics, 26.07.2019 09:40




Mathematics, 26.07.2019 09:40

History, 26.07.2019 09:40



Physics, 26.07.2019 09:40


History, 26.07.2019 09:40



Mathematics, 26.07.2019 09:40



English, 26.07.2019 09:40

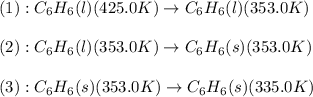
![\Delta H=[m\times c_{p,l}\times (T_{final}-T_{initial})]+m\times \Delta H_{fusion}+[m\times c_{p,s}\times (T_{final}-T_{initial})]](/tpl/images/0499/2996/53889.png)
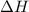 = heat available for the reaction =
= heat available for the reaction = 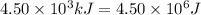
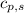 = specific heat of solid benzene =
= specific heat of solid benzene = 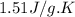
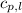 = specific heat of liquid benzene =
= specific heat of liquid benzene = 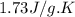
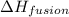 = enthalpy change for fusion =
= enthalpy change for fusion = 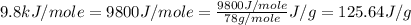
![\Delta H=[125g\times 1.73J/g.K\times (353-425)K]+125g\times -125.64J/g+[125g\times 1.51J/g.K\times (335-353)K]](/tpl/images/0499/2996/d60b8.png)