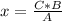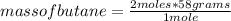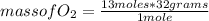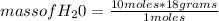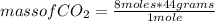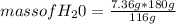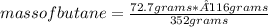Chemistry, 21.08.2019 18:40 serenityarts123
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g). calculate the mass of water produced when 7.36g of butane reacts with excess oxygen.. express your answer to three significant figures and include the appropriate units.. calculate the mass of butane needed to produce 72.7g of carbon dioxide.. express your answer to three significant figures and include the appropriate units.. how do you do it?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1
You know the right answer?
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g). calculate the mass of water produced when 7.36g of butane react...
Questions






Biology, 04.07.2020 23:01





Computers and Technology, 04.07.2020 23:01