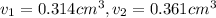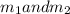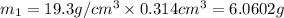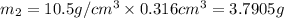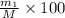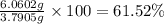Chemistry, 11.11.2019 06:31 kimloveswim
Gold is alloyed(mixed) with other metals to increase its hardness in making jewelry. (a) consider a piece of gold jewelry that weighs 9.85 g and has a volume of 0.675 cm3. the jewelry contains only gold and silver, which have densities of 19.3 g/cm3 and 10.5 g/cm3, repectively. if the total volume of the jewelry is the sum of the volumes of the gold and silver that it contains. calculate the percentage of gold(by mass) in the jewelry. (b) the relative amount of gold in an alloy is commonly expressed in units of karats.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
Gold is alloyed(mixed) with other metals to increase its hardness in making jewelry. (a) consider a...
Questions




History, 20.04.2020 22:12
















Biology, 20.04.2020 22:13

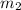
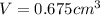
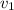
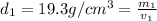
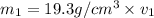
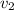
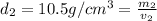
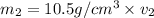
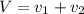
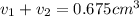 ..(1)
..(1)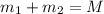
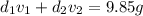
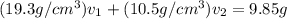 ..(2)
..(2)