Chemistry, 02.02.2020 12:42 DonovanBaily42
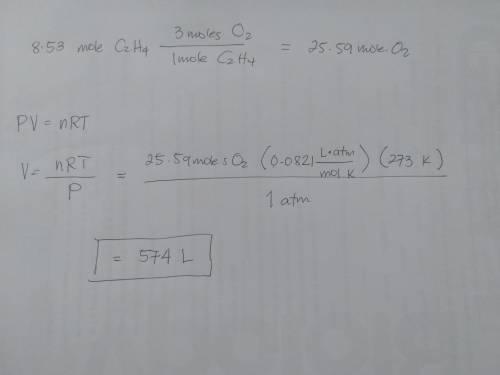
What volume of oxygen at stp is needed to fully react with 8.53 mol of c2h4?
c2h4 reacts with o2, according to the following equation:
c2h4(g) + 3o2(g) → 2co2(g) + 2h2o(g)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
What volume of oxygen at stp is needed to fully react with 8.53 mol of c2h4?
c2h4 reacts with...
c2h4 reacts with...
Questions

Medicine, 15.09.2021 22:20




English, 15.09.2021 22:20


History, 15.09.2021 22:20

Mathematics, 15.09.2021 22:20





Biology, 15.09.2021 22:20

Mathematics, 15.09.2021 22:20

History, 15.09.2021 22:20

Mathematics, 15.09.2021 22:20