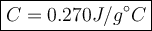2. a 237 g piece of molybdenum, initially at 100.0 °c, was dropped into 244 g of
water at 10.0...

Chemistry, 29.01.2020 14:40 alonnachambon
2. a 237 g piece of molybdenum, initially at 100.0 °c, was dropped into 244 g of
water at 10.0°c. when the system came to thermal equilibrium, the temperature
was 15.3°c. what is the specific heat capacity of molybdenum? the specific heat
capacity of water is 4.184 j/g. k. (2.5 points)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
Questions

Mathematics, 30.12.2019 04:31



Chemistry, 30.12.2019 04:31

Mathematics, 30.12.2019 04:31

Mathematics, 30.12.2019 04:31

Mathematics, 30.12.2019 04:31



Mathematics, 30.12.2019 04:31

Mathematics, 30.12.2019 04:31



Social Studies, 30.12.2019 04:31


Mathematics, 30.12.2019 04:31



English, 30.12.2019 04:31