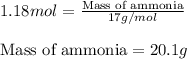Chemistry, 29.01.2020 05:44 amylumey2005
What is the theoretical yield of ammonia (in grams) if 16.55 grams of nitrogen gas and 10.15 grams of hydrogen gas are allowed to react?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3
You know the right answer?
What is the theoretical yield of ammonia (in grams) if 16.55 grams of nitrogen gas and 10.15 grams o...
Questions

English, 13.06.2021 20:20

Business, 13.06.2021 20:20



Medicine, 13.06.2021 20:20

Mathematics, 13.06.2021 20:20

Mathematics, 13.06.2021 20:20

Chemistry, 13.06.2021 20:20



Computers and Technology, 13.06.2021 20:20

Mathematics, 13.06.2021 20:20

Spanish, 13.06.2021 20:20

Mathematics, 13.06.2021 20:20



Physics, 13.06.2021 20:20


Biology, 13.06.2021 20:30

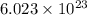 of particles.
of particles.
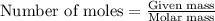 .....(1)
.....(1)
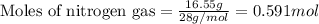
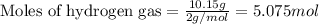
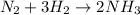
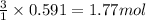 of hydrogen.
of hydrogen.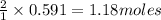 of ammonia
of ammonia