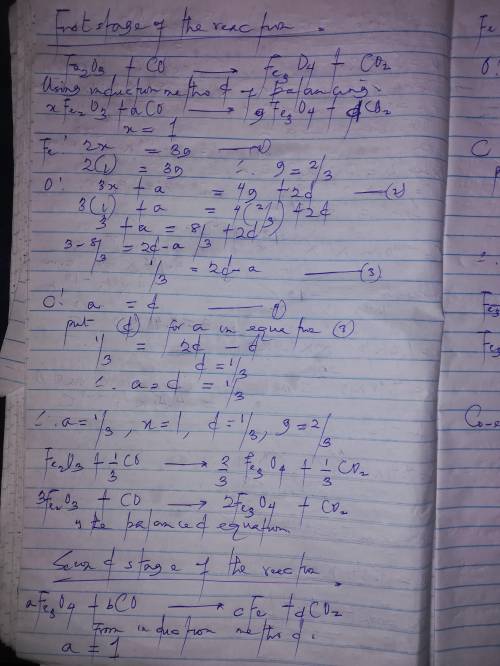In one stage in the commercial production of iron metal in a blast furnace, the iron(iii) oxide (fe2o3) reacts with carbon monoxide to form solid fe3o4 and carbon dioxide gas. in a second stage, the fe3o4 reacts further with carbon monoxide to produce solid elemental iron and carbon dioxide. what is the coefficient of iron(iii) oxide in the second stage of the balanced equations?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
In one stage in the commercial production of iron metal in a blast furnace, the iron(iii) oxide (fe2...
Questions

Mathematics, 18.03.2021 01:20





World Languages, 18.03.2021 01:20

Social Studies, 18.03.2021 01:20

English, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20




Mathematics, 18.03.2021 01:20






Computers and Technology, 18.03.2021 01:20