Chemistry, 29.01.2020 05:46 Ezekielcassese
Acertain liquid x has a normal boiling point of 118.90 °c and a boiling point elevation constant kb = 0.82 °c*kg*mol^-1. calculate the boiling point of a solution made of 54.g of potassium bromide (kbr) dissolved in 750. g of x. be sure your answer is rounded to the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3


Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
Acertain liquid x has a normal boiling point of 118.90 °c and a boiling point elevation constant kb...
Questions

History, 01.02.2021 14:30

English, 01.02.2021 14:30

Mathematics, 01.02.2021 14:30

Mathematics, 01.02.2021 14:30






English, 01.02.2021 14:30

Mathematics, 01.02.2021 14:30

Mathematics, 01.02.2021 14:30

Mathematics, 01.02.2021 14:30

Mathematics, 01.02.2021 14:30

Chemistry, 01.02.2021 14:30

English, 01.02.2021 14:30

Geography, 01.02.2021 14:40



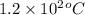
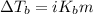
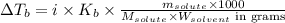
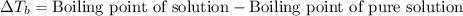
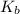 = molal boiling point elevation constant = 0.82°C/m
= molal boiling point elevation constant = 0.82°C/m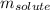 = Given mass of solute (potassium bromide) = 54. g
= Given mass of solute (potassium bromide) = 54. g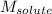 = Molar mass of solute (potassium bromide) = 119 g/mol
= Molar mass of solute (potassium bromide) = 119 g/mol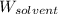 = Mass of solvent (liquid X) = 750. g
= Mass of solvent (liquid X) = 750. g