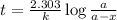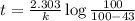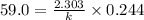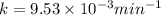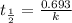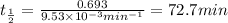Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2


Chemistry, 23.06.2019 10:30
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
You know the right answer?
After 59.0 min, 59.0 min, 43.0 % 43.0% of a compound has decomposed. what is the half‑life of this r...
Questions


Mathematics, 18.10.2019 15:30

Mathematics, 18.10.2019 15:30

English, 18.10.2019 15:30


History, 18.10.2019 15:30


English, 18.10.2019 15:30

Mathematics, 18.10.2019 15:30

Physics, 18.10.2019 15:30

Health, 18.10.2019 15:30

Mathematics, 18.10.2019 15:30

Mathematics, 18.10.2019 15:30

Social Studies, 18.10.2019 15:30


English, 18.10.2019 15:30


Mathematics, 18.10.2019 15:30