Consider this reaction:
2cl2o5 (g) → 2cl2 (g) + 5o2 (g)
at a certain temp...

Consider this reaction:
2cl2o5 (g) → 2cl2 (g) + 5o2 (g)
at a certain temperature it obeys this rate law.
rate = (6.48 m-1 • s-1)[cl2o5]2
suppose a vessel contains cl2o5 at a concentration of 1.16 m. calculate the concentration of cl2o5 in the vessel 0.820 seconds later. you may assume no other reaction is important.
round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
Questions

Mathematics, 31.08.2019 14:30

Mathematics, 31.08.2019 14:30



Mathematics, 31.08.2019 14:30


Mathematics, 31.08.2019 14:30

Biology, 31.08.2019 14:30


Geography, 31.08.2019 14:30

Mathematics, 31.08.2019 14:30



Mathematics, 31.08.2019 14:30




Mathematics, 31.08.2019 14:30


Mathematics, 31.08.2019 14:30

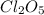 in the vessel 0.820 seconds later is, 0.16 M
in the vessel 0.820 seconds later is, 0.16 M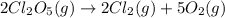
![rate=(6.48M^{-1}s^{-1})[Cl_2O_5]^2](/tpl/images/0479/5701/ef798.png)
![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0479/5701/ccade.png)
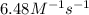
![[A_t]](/tpl/images/0479/5701/5262c.png) = final concentration = ?
= final concentration = ?![[A_o]](/tpl/images/0479/5701/dc622.png) = initial concentration = 1.16 M
= initial concentration = 1.16 M![6.48\times 0.820=\frac{1}{[A_t]}-\frac{1}{1.16}](/tpl/images/0479/5701/3da76.png)
![[A_t]=0.16M](/tpl/images/0479/5701/3ee9b.png)


