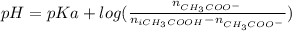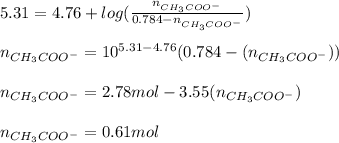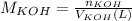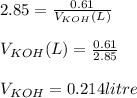Chemistry, 29.01.2020 01:45 jacksonyodell8601
You need to prepare an acetate buffer of ph 5.31 5.31 from a 0.784 m 0.784 m acetic acid solution and a 2.85 m koh 2.85 m koh solution. if you have 930 ml 930 ml of the acetic acid solution, how many milliliters of the koh koh solution do you need to add to make a buffer of ph 5.31 5.31 ? the p k a pka of acetic acid is 4.76. 4.76.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
You need to prepare an acetate buffer of ph 5.31 5.31 from a 0.784 m 0.784 m acetic acid solution an...
Questions

Spanish, 03.11.2019 07:31


Physics, 03.11.2019 07:31

History, 03.11.2019 07:31


Mathematics, 03.11.2019 07:31


Mathematics, 03.11.2019 07:31



Mathematics, 03.11.2019 07:31

Spanish, 03.11.2019 07:31

Mathematics, 03.11.2019 07:31


History, 03.11.2019 07:31

Biology, 03.11.2019 07:31

Mathematics, 03.11.2019 07:31



![pH=pKa + log(\frac{[CH_{3}COO^{-}]}{[CH_{3}COOH]})](/tpl/images/0479/5908/7f7e8.png)