
Chemistry, 29.01.2020 01:47 Buttercream16
Calculate the ph of sweater which has a hydrogen ion concentration of 1 times 10 to the power of 8

Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:02
1.milk of magnesia, a suspension of mg(oh)2in water, reacts with stomach acid (hcl) in a neutralization reaction. mg(oh)2(s) + 2 hcl(aq) −→ 2 h2o(l) + mgcl2(aq) what mass of mgcl2 will be produced if 5.49 g of mg(oh)2 reacts? answer in units of g. 2.what mass of hcl is required to completely react with 5.49 g of mg(oh)2? answer in units of g.
Answers: 3


Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 10:30
If a computer chip switches off -on-off in 0.015 us, what is the switching time in nanoseconds?
Answers: 2
You know the right answer?
Calculate the ph of sweater which has a hydrogen ion concentration of 1 times 10 to the power of 8...
Questions

Mathematics, 04.08.2019 16:30


English, 04.08.2019 16:30


History, 04.08.2019 16:30

English, 04.08.2019 16:30




Social Studies, 04.08.2019 16:30

Biology, 04.08.2019 16:30

History, 04.08.2019 16:30

History, 04.08.2019 16:30

Chemistry, 04.08.2019 16:30

Business, 04.08.2019 16:30

Biology, 04.08.2019 16:30


History, 04.08.2019 16:30

Biology, 04.08.2019 16:30

![[H^{+}]=1\times 10^{-8}](/tpl/images/0479/6062/852ba.png) is
is 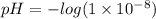

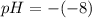
![[H^{+}]=1\times 10^{8}](/tpl/images/0479/6062/abc48.png)


