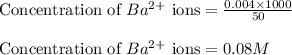Chemistry, 29.01.2020 00:48 brooke3493
A20.0 ml sample of 0.200 m k₂co₃ solution is added to 30.0 ml of 0.400 m ba(no₃)₂ solution. barium carbonate precipitates.
the concentration of barium ion, ba²⁺, in solution after the reaction is

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
You know the right answer?
A20.0 ml sample of 0.200 m k₂co₃ solution is added to 30.0 ml of 0.400 m ba(no₃)₂ solution. barium c...
Questions

Social Studies, 31.08.2019 21:30

Mathematics, 31.08.2019 21:30

Geography, 31.08.2019 21:30

Computers and Technology, 31.08.2019 21:30

Geography, 31.08.2019 21:30

Social Studies, 31.08.2019 21:30

Mathematics, 31.08.2019 21:30

Biology, 31.08.2019 21:30



Mathematics, 31.08.2019 21:30



English, 31.08.2019 21:30

World Languages, 31.08.2019 21:30


Physics, 31.08.2019 21:30

Physics, 31.08.2019 21:30


History, 31.08.2019 21:30

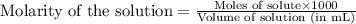 .....(1)
.....(1)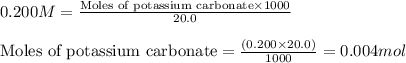
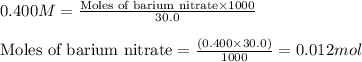
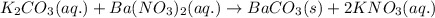
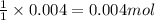 of barium nitrate
of barium nitrate