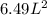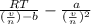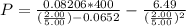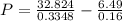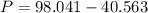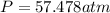Chemistry, 28.01.2020 23:45 marinahuntg
What would be the pressure of a 5.00 mol sample of cl₂ at 400.0 k in a 2.00 l container found using the van der waals equation? for cl₂, a = 6.49 l²・atm/mol² and b = 0.0652 l/mol.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1


Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
What would be the pressure of a 5.00 mol sample of cl₂ at 400.0 k in a 2.00 l container found using...
Questions



Mathematics, 28.02.2020 23:56


Mathematics, 28.02.2020 23:56


Mathematics, 28.02.2020 23:56