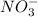Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
For each of the following compounds, state whether it is ionic or covalent, and if it is ionic, writ...
Questions

Mathematics, 09.11.2020 22:10



Computers and Technology, 09.11.2020 22:10




History, 09.11.2020 22:10


Health, 09.11.2020 22:10



Spanish, 09.11.2020 22:10


History, 09.11.2020 22:10





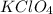
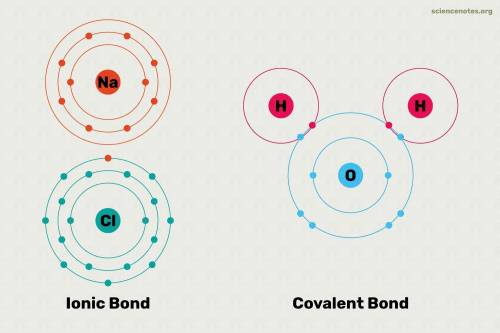
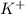 which is a metal and
which is a metal and 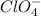 ion which is a polyatomic ion. Thus, it will form an ionic compound.
ion which is a polyatomic ion. Thus, it will form an ionic compound.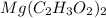
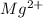 which is a metal and
which is a metal and 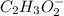 ion which is a polyatomic ion. Thus, it will form an ionic compound.
ion which is a polyatomic ion. Thus, it will form an ionic compound.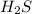
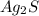
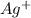 which is a metal and sulfur,
which is a metal and sulfur, 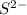 which is a non-metal. Thus, it will form an ionic compound.
which is a non-metal. Thus, it will form an ionic compound.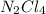
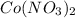
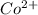 which is a metal and
which is a metal and 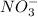 ion which is a polyatomic ion. Thus, it will form an ionic compound.
ion which is a polyatomic ion. Thus, it will form an ionic compound.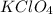 is an ionic compound. The ions =
is an ionic compound. The ions = 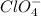
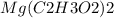 is an ionic compound. The ions =
is an ionic compound. The ions = 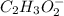
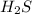 is a covalent compound formed by two non-metals ( hydrogen and sulfur )
is a covalent compound formed by two non-metals ( hydrogen and sulfur )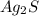 is an ionic compound. The ions =
is an ionic compound. The ions = 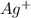 and
and 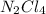 is a covalent compound formed by Nitrogen and Chlorine ( Non-metals )
is a covalent compound formed by Nitrogen and Chlorine ( Non-metals )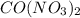 is an ionic compound. The ions =
is an ionic compound. The ions = 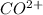 and
and