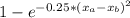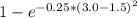Chemistry, 28.01.2020 20:48 Tabbicat021
The compound aluminum nitride () is a compound semiconductor having mixed ionic and covalent bonding. the electronegativities for and are 1.5 and 3.0 respectively. calculate the fraction of the bonding that is ionic. (enter your answer to three significant figures.) =

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2


Chemistry, 23.06.2019 05:00
110 g of water (specific heat = 4.184 j/g c) and 100 g of a metal sample (specific heat = 0.397 j/g c) are heated from 25 degrees c to 75 degrees c. which substance required more thermal energy?
Answers: 1
You know the right answer?
The compound aluminum nitride () is a compound semiconductor having mixed ionic and covalent bonding...
Questions







Social Studies, 15.12.2020 19:30


History, 15.12.2020 19:30


Mathematics, 15.12.2020 19:30







History, 15.12.2020 19:30

Mathematics, 15.12.2020 19:30