
Chemistry, 28.01.2020 20:51 queenkimm26
Assume that each atom is a sphere, and that the surface of each atom is in contact with its nearest neighbor. determine the percentage of unit cell volume that is occupied in (a) a face- centered cubic lattice, (b) a body-centered cubic lattice, and (c) a diamond lattice.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2

Chemistry, 23.06.2019 06:30
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3
You know the right answer?
Assume that each atom is a sphere, and that the surface of each atom is in contact with its nearest...
Questions

Mathematics, 19.03.2021 01:30

History, 19.03.2021 01:30





History, 19.03.2021 01:30


Social Studies, 19.03.2021 01:30






Mathematics, 19.03.2021 01:30

Mathematics, 19.03.2021 01:30


Mathematics, 19.03.2021 01:30


Social Studies, 19.03.2021 01:30

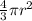
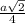
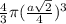 =
= 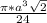
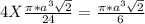
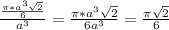 = 0.7405
= 0.7405 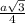
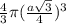 =
=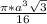
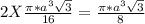
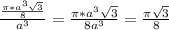 = 0.6803
= 0.6803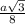
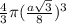 =
= 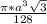
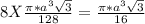
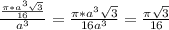 = 0.3401
= 0.3401

