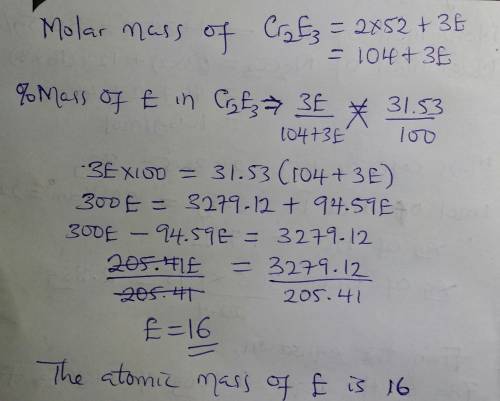Chemistry, 28.01.2020 19:41 izzy201995
Abinary compound created by reaction of chromium and an unknown element e contains 68.47% cr and 31.53% e by mass. if the formula of the compound is cr2e3, calculate the atomic mass of e.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2


Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1
You know the right answer?
Abinary compound created by reaction of chromium and an unknown element e contains 68.47% cr and 31....
Questions



Computers and Technology, 30.11.2019 00:31


Mathematics, 30.11.2019 00:31

Mathematics, 30.11.2019 00:31



Physics, 30.11.2019 00:31





Mathematics, 30.11.2019 00:31




Mathematics, 30.11.2019 00:31