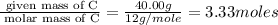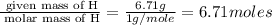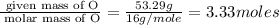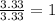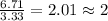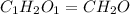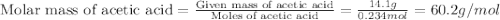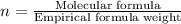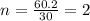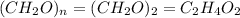Chemistry, 28.01.2020 19:48 kaylallangari549
Acetic acid is an organic compound composed of 40.00% c, 6.71% h, and the rest oxygen. if 0.234 mol of acetic acid has a mass of 14.1 g, what are the empirical and molecular formulas of acetic acid?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
Acetic acid is an organic compound composed of 40.00% c, 6.71% h, and the rest oxygen. if 0.234 mol...
Questions

English, 02.06.2021 16:10


Biology, 02.06.2021 16:10



Mathematics, 02.06.2021 16:10



Mathematics, 02.06.2021 16:10

English, 02.06.2021 16:10


English, 02.06.2021 16:10

History, 02.06.2021 16:10






Mathematics, 02.06.2021 16:10

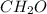 and
and 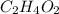 respectively.
respectively.