
Chemistry, 28.01.2020 08:31 ketricduggerp2ciuc
An old (pre-1987) penny is nearly pure copper. if such a penny has a mass of 3.3 g, how many moles of copper atoms would be on one penny?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
An old (pre-1987) penny is nearly pure copper. if such a penny has a mass of 3.3 g, how many moles o...
Questions




Arts, 20.09.2020 18:01

Mathematics, 20.09.2020 18:01


Computers and Technology, 20.09.2020 18:01


Geography, 20.09.2020 18:01

Mathematics, 20.09.2020 18:01


History, 20.09.2020 18:01


History, 20.09.2020 18:01

History, 20.09.2020 18:01


Computers and Technology, 20.09.2020 18:01

Mathematics, 20.09.2020 18:01


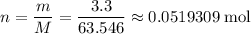 .
.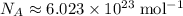 .
.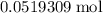 copper atoms, the number of copper atoms would be
copper atoms, the number of copper atoms would be 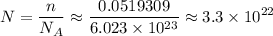 .
.


