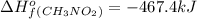Top fuel dragsters and funny cars burn nitro-methane as fuel according to the following balanced combustion equation: 2ch3no2(l)+3/2o2(g)→2co2(g)+3h2o(g) +n2(g). the standard enthalpy of combustion for nitromethane is −709.2kj/mol. calculate the standard enthalpy of formation(delta h formation) for nitro-methane.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2
You know the right answer?
Top fuel dragsters and funny cars burn nitro-methane as fuel according to the following balanced com...
Questions




Mathematics, 22.02.2021 14:00


History, 22.02.2021 14:00

Mathematics, 22.02.2021 14:00



English, 22.02.2021 14:00

English, 22.02.2021 14:00



Social Studies, 22.02.2021 14:00

Mathematics, 22.02.2021 14:00

Mathematics, 22.02.2021 14:00




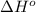
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0475/2966/45485.png)
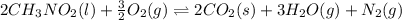
![\Delta H^o_{rxn}=[(n_{(CO_2)}\times \Delta H^o_f_{(CO_2)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})+(n_{(N_2)}\times \Delta H^o_f_{(N_2)})]-[(n_{(CH_3NO_2)}\times \Delta H^o_f_{(CH_3NO_2)})+(n_{(O_2)}\times \Delta H^o_f_{(O_2)})]](/tpl/images/0475/2966/ea72d.png)
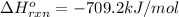
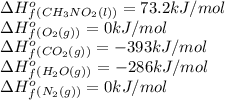
![-709.2=[(2\times -393)+(3\times -286)+(1\times 0)]-[(2\times \Delta H^o_f_{(CH_3NO_2)})+(\frac{3}{2}\times 0)]](/tpl/images/0475/2966/4d16f.png)