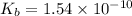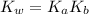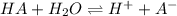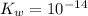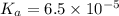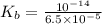Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
The acid-dissociation constant, ka, for benzoic acid is 6.5 × 10-5. which will you use to calculate...
Questions


Mathematics, 24.06.2019 06:10

Mathematics, 24.06.2019 06:10


Mathematics, 24.06.2019 06:10

Health, 24.06.2019 06:10

Health, 24.06.2019 06:10


Mathematics, 24.06.2019 06:10

English, 24.06.2019 06:10

Geography, 24.06.2019 06:10


Mathematics, 24.06.2019 06:20

Chemistry, 24.06.2019 06:20

Mathematics, 24.06.2019 06:20



Mathematics, 24.06.2019 06:20

Chemistry, 24.06.2019 06:20