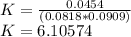Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1
You know the right answer?
Asolution is made by mixing 30.0 ml of 0.150 m compound a with 25.0 ml of 0.200 m compound b. at equ...
Questions



Chemistry, 25.05.2021 18:10

Mathematics, 25.05.2021 18:10

Law, 25.05.2021 18:10

Mathematics, 25.05.2021 18:10




Chemistry, 25.05.2021 18:10





Arts, 25.05.2021 18:10

Mathematics, 25.05.2021 18:10


History, 25.05.2021 18:10

Mathematics, 25.05.2021 18:10

Mathematics, 25.05.2021 18:10

![K=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0474/5763/91a0a.png)
![K=\frac{[C]^}{[A][B]}](/tpl/images/0474/5763/1e2ca.png)
![[A]=\frac{(0.030L)*(0.150)}{0.03+0.025}=0.0818M](/tpl/images/0474/5763/9dcbf.png)
![[B]=\frac{(0.025L)*(0.200)}{0.03+0.025}=0.0909M](/tpl/images/0474/5763/9e9a1.png)
![[C]=0.0454 M](/tpl/images/0474/5763/82746.png)