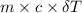The combustion of palmitic acid in a bomb calorimeter yields energy in the form of heat released upon oxidation. from a thermodynamic perspective and with respect to the calorimeter experiment, what would you expect the combustion of the same amount of palmitic acid in our body to yield? a. less energyb. more energyc. the same amount of energyd. the calorimeter experiment is irrelevant to the combustion of palmitic acid in the human body.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3


Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
The combustion of palmitic acid in a bomb calorimeter yields energy in the form of heat released upo...
Questions

Mathematics, 15.12.2020 21:40



Physics, 15.12.2020 21:40

Mathematics, 15.12.2020 21:40

History, 15.12.2020 21:40

Biology, 15.12.2020 21:40

Mathematics, 15.12.2020 21:40


Social Studies, 15.12.2020 21:40


Mathematics, 15.12.2020 21:40

History, 15.12.2020 21:40

Biology, 15.12.2020 21:40


Mathematics, 15.12.2020 21:40

Arts, 15.12.2020 21:40

Arts, 15.12.2020 21:40


English, 15.12.2020 21:40