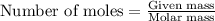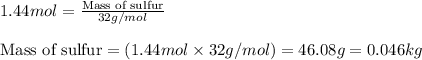Chemistry, 28.01.2020 00:31 lolfunny124
Sulfuric acid is essential to dozens of important industries from steelmaking to plastics and pharmaceuticals. more sulfuric acid is made than any other industrial chemical, and world production exceeds per year. the first step in the synthesis of sulfuric acid is usually burning solid sulfur to make sulfur dioxide gas. suppose an engineer studying this reaction introduces of solid sulfur and of oxygen gas at into an evacuated tank. the engineer believes for the reaction at this temperature. calculate the mass of solid sulfur he expects to be consumed when the reaction reaches equilibrium. round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
Sulfuric acid is essential to dozens of important industries from steelmaking to plastics and pharma...
Questions



Arts, 16.10.2020 20:01

Mathematics, 16.10.2020 20:01

Mathematics, 16.10.2020 20:01


Biology, 16.10.2020 20:01


Mathematics, 16.10.2020 20:01

Computers and Technology, 16.10.2020 20:01





Mathematics, 16.10.2020 20:01

Mathematics, 16.10.2020 20:01




Mathematics, 16.10.2020 20:01

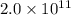 per year.
per year. 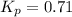 for the reaction at this temperature.
for the reaction at this temperature. 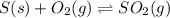
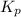 for above equation follows:
for above equation follows: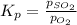
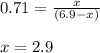
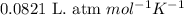
![950^oC=[950+273]K=1223K](/tpl/images/0474/1207/8397a.png)
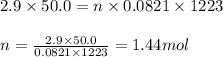
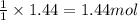 of sulfur
of sulfur