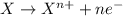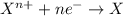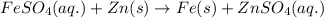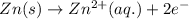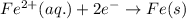Chemistry, 28.01.2020 00:31 leianagaming
Study this chemical reaction: feso4 (aq) + zn (s) --> fe (s) + znso4 (aq) then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction.
oxidation:
reduction:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 23.06.2019 09:10
Complete the following radioactive decay problem. tan+on-? c+th
Answers: 1
You know the right answer?
Study this chemical reaction: feso4 (aq) + zn (s) --> fe (s) + znso4 (aq) then, write balanced...
Questions

Mathematics, 25.01.2021 23:30

Mathematics, 25.01.2021 23:30


English, 25.01.2021 23:30



Mathematics, 25.01.2021 23:30


English, 25.01.2021 23:30


Mathematics, 25.01.2021 23:30



Mathematics, 25.01.2021 23:30


English, 25.01.2021 23:30



English, 25.01.2021 23:30