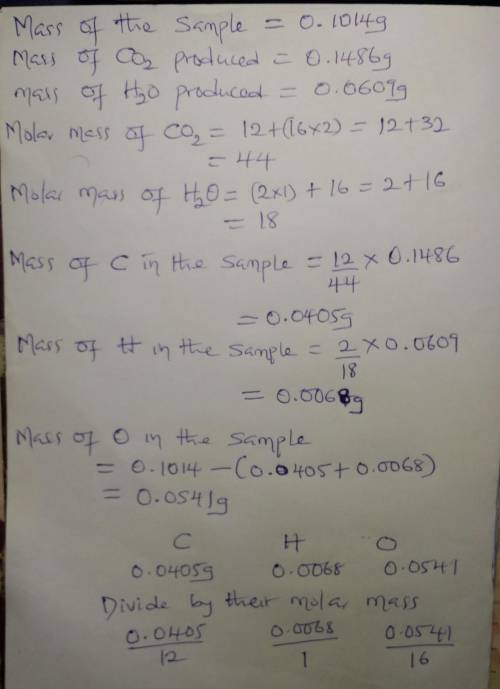Chemistry, 28.01.2020 00:31 girlygirl2007
A0.1014 g sample of a purified cho compound was burned in a combustion apparatus and produced 0.1486 g co2 and 0.0609 g of h2o. what is the empirical formula of this cho compound? enter as c#h#o#, e. g. c2h3o2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1


Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
A0.1014 g sample of a purified cho compound was burned in a combustion apparatus and produced 0.1486...
Questions

Spanish, 24.11.2020 19:00


Health, 24.11.2020 19:00

History, 24.11.2020 19:00

Law, 24.11.2020 19:00



Mathematics, 24.11.2020 19:00



Mathematics, 24.11.2020 19:00



Mathematics, 24.11.2020 19:00


Business, 24.11.2020 19:00

History, 24.11.2020 19:00


Spanish, 24.11.2020 19:00

Mathematics, 24.11.2020 19:00