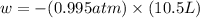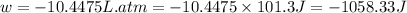Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1


Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Calculate the expansion work done against a constant external pressure of 0.995 atm and at a constan...
Questions


Mathematics, 20.12.2021 01:00



SAT, 20.12.2021 01:00




Computers and Technology, 20.12.2021 01:00




SAT, 20.12.2021 01:00

SAT, 20.12.2021 01:00

Physics, 20.12.2021 01:00


World Languages, 20.12.2021 01:00

Computers and Technology, 20.12.2021 01:00

English, 20.12.2021 01:00

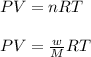
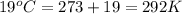
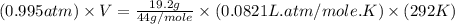
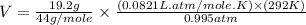
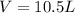
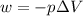
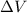 = volume = 10.5 L
= volume = 10.5 L