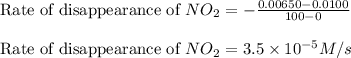Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1

Chemistry, 21.06.2019 19:00
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3
You know the right answer?
Nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2no2 → 2no + o2 in a parti...
Questions















Business, 24.12.2019 17:31





Computers and Technology, 24.12.2019 17:31

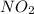 is
is 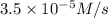
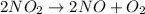
![\text{Rate of disappearance of }NO_2=-\frac{\Delta [NO_2]}{\Delta t}](/tpl/images/0473/6156/ea698.png)
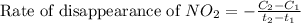
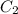 = final concentration of
= final concentration of 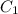 = initial concentration of
= initial concentration of 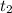 = final time = 100 minutes
= final time = 100 minutes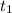 = initial time = 0 minutes
= initial time = 0 minutes